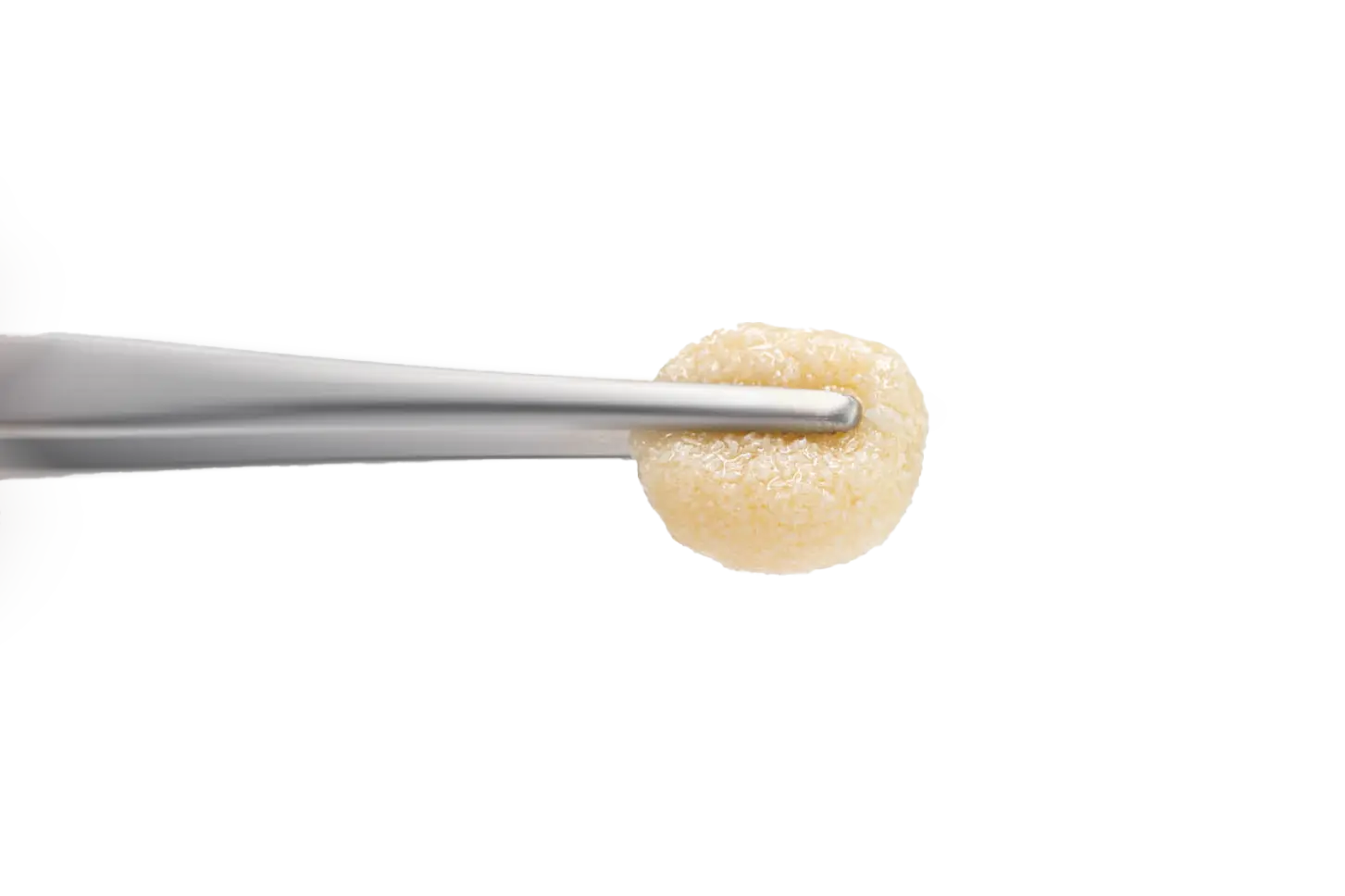
maxgraft® +HyA
Processed allograft with hyaluronate

Improved handling
Enhanced bone regeneration
Favourable soft tissue healing
maxgraft®+HyA extends the well-established maxgraft® portfolio by combining allogenic granules with hyaluronate. Due to the extraordinary liquid-binding properties of hyaluronate, maxgraft®+HyA allows for seamless preparation of sticky bone upon hydration. The combination of maxgraft®’s high biological regenerative potential and complete remodeling with the unique benefits of hyaluronate enhances clinical outcomes and supports soft tissue healing.1-3
Clinical versatility with four solutions
Pure cancellous maxgraft®+HyA (small and large granules) provides a highly porous scaffold that supports optimal revascularization and the supply of vital cells, enabling the rapid regeneration of vital bone tissue.4 The cortico-cancellous variant (small granules), composed of approx. 70% cortical and 30% cancellous bone particulate, offers enhanced volume stability. This makes it particularly beneficial for guided bone regeneration in the aesthetic zone, where predictable volume maintenance and soft tissue integration are essential. The extra-small cortico-cancellous variant (XS granules) forms a paste-like consistency upon hydration, allowing for easy application via a conventional Luer-tip syringe—ideal for grafting small or narrow defects.
IMPLANTOLOGY, PERIODONTOLOGY, ORAL AND CMF SURGERY
- Periodontal osseous defects
- Regeneration of extraction sockets (socket preservation)
- Regeneration of missing bone tissue around dental implants
- Regeneration of gaps around block grafts
- Sinus augmentation
- Horizontal and vertical augmentation
Application
maxgraft®+HyA requires hydration before use (approx. 0.8 ml saline solution or patient blood per 1 ml maxgraft®+HyA), which can be conveniently performed in the provided jar. Detailed recommendations on hydration volumes, considering the composition of the different variants, can be found in the user manual.
Hydration
Add the liquid for hydration dropwise and adjust the consistency as preferred. Use a 1 ml syringe to measure the volume of liquid for hydration in order to achieve consistent outcomes. Due to maxgraft®+HyA’s ability to rapidly uptake blood from the defect site, it is recommended to aim for a relatively dry consistency when hydrating the material, rather than a runny one.
Mixing with autologous bone
When working with autologous bone, the bone chips should be added to maxgraft®+HyA prior to hydration. The required amount of liquid may differ from the hydration protocol and should be determined by careful dropwise addition of the liquid.
XS-Variant
The extra small (XS) variant (granules < 0.25 mm) forms a past-like consistency upon hydration and can be then transferred into a conventional 5-6 ml Luer-tip syringe for precise application in small defects. Additionally, a needle* can be attached to facilitate the placement of the bone graft in narrow defects, such as gaps around implants and periodontal defects.
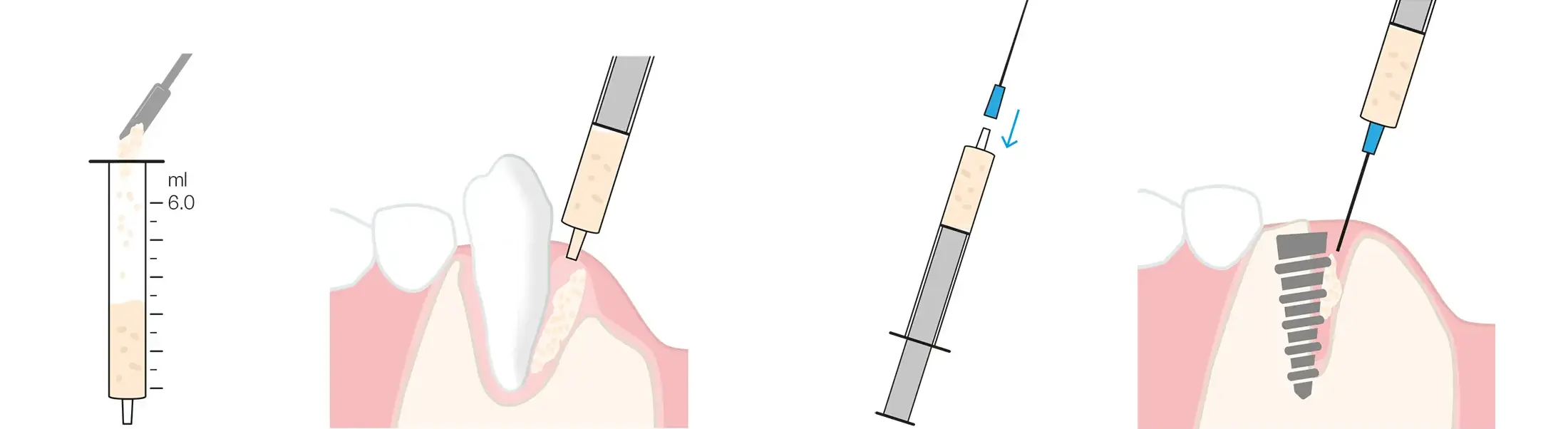
* Recommended size: 14G/ø 2.1 mm. Example of a suitable needle: transcodent™ Dental Needle 2.1 x 39 mm. Art. No. 162062
Fixation
Thorough fixation with a barrier membrane supports the stabilization of the bone grafting material at the application site. Moreover, in order to prevent ingrowth of soft tissue and to allow for undisturbed healing, the augmentation area should be covered with a barrier membrane according to the principles of GBR.
Using the botiss grafter
The botiss grafter enables convenient hydration and efficient application of maxgraft®+HyA (small and large variants). It also allows the application of mixtures of maxgraft® +HyA and autologous bone or cerabone®. For homogeneous distribution, the materials should be mixed in the desired ratio before filling into the applicator. See how it works
- Improved handling properties1,2
- Precise application to the defect site2
- Enhanced bone regeneration 3
- Favorable soft tissue healing1
- Natural bone structure of bone graft with preserved collagen content5,6
- Controlled graft remodeling into native bone4,7
You are currently viewing a placeholder content from Vimeo. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationProduct Specifications
maxgraft® +HyA cancellous granules
| Art.-No. | maxgraft® Particle Size | Content |
|---|---|---|
| 38005S | 0.25 – 1.0 mm | 1 x 0.5 ml |
| 38010S | 0.25 – 1.0 mm | 1 x 1 ml |
| 38020S | 0.25 – 1.0 mm | 1 x 2 ml |
| 38005L | 1.0 – 2.0 mm | 1 x 0.5 ml |
| 38010L | 1.0 – 2.0 mm | 1 x 1 ml |
| 38020L | 1.0 – 2.0 mm | 1 x 2 ml |
maxgraft® +HyA cortico-cancellous granules
| Art.-No. | maxgraft® Particle Size | Content |
|---|---|---|
| 34005XS | < 0,25 mm | 1 x 0.5 ml |
| 34010XS | < 0,25 mm | 1 x 1 ml |
| 34005S | 0.25 – 1.0 mm | 1 x 0.5 ml |
| 34010S | 0.25 – 1.0 mm | 1 x 1 ml |
| 34020S | 0.25 – 1.0 mm | 1 x 2 ml |
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
Related Products

cerabone® plus
Natural bovine bone substitute material with hyaluronate
Combines the established bovine bone grafting material cerabone® with the well-known properties of hyaluronic acid.
maxgraft® granules
Processed human allograft
Allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process