maxgraft® blocks
Processed human allograft

natural bone regeneration
ALTERNATIVE TO PATIENT’S OWN BONE
SHORTER TREATMENT TIMES
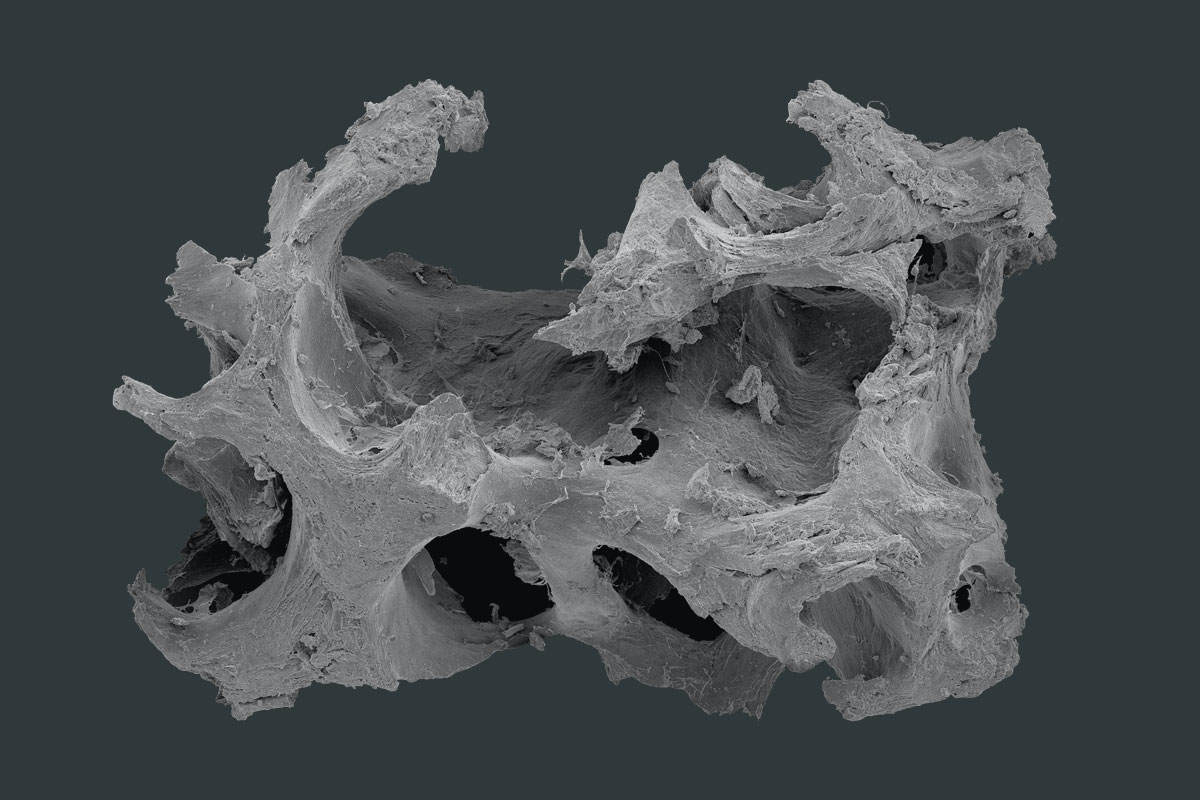
maxgraft® blocks are allogenic bone grafts from human donor bone, processed by the Cells+Tissuebank Austria in a special purification process (Allotec® process) and are available in cancellous and cortico-cancellous forms. Due to the preserved bone structure and collagen content of human bone, maxgraft® blocks serve as a osteoconductive scaffold for natural bone regeneration and have the potential to be completely remodeled into the patient’s own bone1.
ALLOGENIC ALTERNATIVE
The cancellous structure of maxgraft® enables optimal revascularization and supply of vital cells and thus rapid regeneration of vital bone tissue2,3. With maxgraft® uni-cortical blocks, greater volume stability is provided due to a compact and dense bone part on one side. maxgraft® blocks are a valid alternative to autologous bone harvesting for bone augmentation procedures1. The harvesting morbidity of a second surgical site and the associated risks of infection, postoperative pain and the risk of loss of bone stability at the harvesting site can be avoided or prevented. For experienced oral and maxillofacial surgeons, customized allogenic bone blocks, maxgraft® bonebuilder, are an additional treatment option to avoid time-consuming block design4.
HIGH PATIENT ACCEPTANCE – SHORTER TREATMENT TIMES
Due to its high biological regenerative capacity and complete remodeling, maxgraft® represents an alternative to the patient’s own bone. The need for a second surgical site is eliminated, surgical time is shortened, and postoperative pain and morbidity for the patient are significantly reduced.
- Reliable, effective alternative to traditional block augmentation techniques with autologous blocks
- Three-dimensional (horizontal and/or vertical) alveolar ridge augmentation
Rehydration
The maxgraft® production process preserves the natural collagen and a residual moisture of <10%. Nevertheless, rehydration is recommended (10 minutes in physiological saline solution) to improve the adaptability of the material to the defect, especially for large blocks. maxgraft® blocks can also be briefly rehydrated in a disposable syringe under vacuum in physiological saline solution (technique according to Dr. Blume).
Fixation – Lag screw technique
maxgraft® blocks must be stabilized to avoid any micro-movement and thus ensure good healing. Direct contact between the local bone and the maxgraft® block ensures safe incorporation and rapid regeneration. With the lag screw technique, a gliding hole is drilled into the bone block (drill diameter corresponds to screw diameter), while a drill with a minimally smaller diameter is used for the local bone. Before fixation, a suitable head space for the screws should be created, e.g. with a diamond-coated ball burr, to avoid soft tissue irritation due to protruding screw heads. Flat-headed osteosynthesis screws are recommended to avoid sharp edges.
Combination with cerabone® or maxgraft® granules
Particulate bone substitute material (e.g. cerabone® or maxgraft® granules) can be used at the edges of the maxgraft® block for contouring. Volume-stable bone substitute materials such as cerabone® can also be used as resorption protection, e.g. especially in the esthetic zone.
Guided Bone Regeneration – Use of a barrier membrane
maxgraft® blocks should be covered with a membrane with sufficient long barrier function (e.g. Jason® membrane) to achieve guided bone regeneration and prevent soft tissue ingrowth.
Wound closure
Consideration of the soft tissue situation prior to surgery is necessary. Proper soft tissue management is critical to the success of the surgical procedure. Tension-free wound closure and adequate soft tissue management help to significantly reduce the risk of complications such as dehiscence. Overlapping mobilization of the soft tissue before suturing should be possible.
Reentry
The healing time for maxgraft® blocks is approx. six months (depending on the location, geometry and extent of the defect). The appropriate time for reentry is determined individually by the practitioner. A radiological control scan is recommended prior to reentry. During reentry, the osteosynthesis screws are removed.
- Preserved trabecular structure of human bone
- Osteoconductive properties that support natural and controlled remodeling
- Natural mineralized collagen
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
CASES
- Dr. Ross CuttsUnited Kingdom
Science
Education
Edutainment
- Dr. Hassan MaghairehUnited Kingdom
RELATED PRODUCTS
maxgraft® granules
Processed human allograft
Allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process

maxgraft® cortico
Bone augmentation with the shell technique
A prefabricated cortical bone strut from human donor bone.
maxgraft® bonebuilder
Customized allogenic bone block
A cancellous allogenic bone block made from human donor bone, individually adapted to the patient defect, which is prepared by Cells+Tissuebank Austria in a special purification process.


























