maxgraft® granules
PROCESSED HUMAN ALLOGRAFT

natural bone regeneration
SHORTER TREATMENT TIMES
EARLIER REENTRY
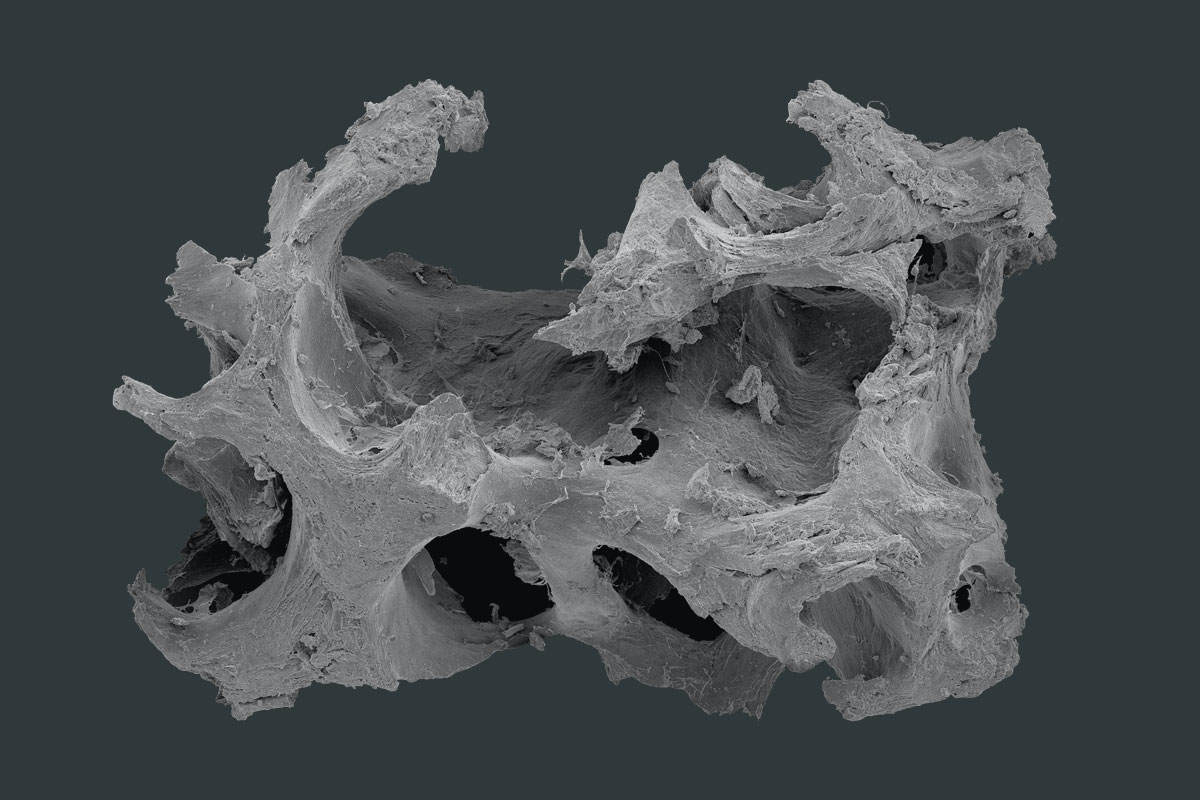
maxgraft® granules are allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process (Allotec® process) and available in cancellous and cortico-cancellous form. Due to its preserved natural bone structure and collagen content, it serves as a scaffold for natural bone regeneration and has the potential of complete remodeling into patients’ own bone 1,2.
FAST REGENERATION
The cancellous structure allows an optimal revascularization and supply of vital cells and therefore enables a rapid regeneration of vital bone tissue1. By adding a compact and dense bone ratio to the cancellous granules (maxgraft® cortico-cancellous granules) a greater volume stability is given, which is useful in cases of for example off-contour augmentations. Depending on the defect size, maxgraft® granules will be stably incorporated within 3–4 months and enable therefore an earlier re-entry in comparison to other grafting materials 2.
HIGH PATIENT ACCEPTANCE – SHORTER TREATMENT TIMES
Due to its high biological regenerative capacity and complete remodeling, maxgraft® represents an alternative to the patient’s own bone. The need for a second surgical site is eliminated, surgical time is shortened, and postoperative pain and morbidity for the patient are significantly reduced.
IMPLANTOLOGY, PERIODONTOLOGY, ORAL AND CMF SURGERY
- Regeneration of extraction sockets (Socket Preservation)
- Regeneration of missing bone tissue around dental implants
- Sinus augmentation
- Regeneration of periodontal bone defects
- Three-dimensional (horizontal and/or vertical) ridge augmentation
- Bony defects
Rehydration
The processing of maxgraft® products preserves the natural collagen content and a residual moisture of <10%. Thus, a rehydration is not mandatory. However, rehydration in blood or saline solution can facilitate the handling and the insertion of maxgraft® granules due to better sticking together.
Application
Compression of the particles should be avoided. Looser particles provide more space for blood vessel ingrowth and new bone formation.
Mixing with autologous bone
Mixing maxgraft® granules with autologous bone increases biological activity in the defect site and contributes to a more rapid regeneration and new bone formation.
Mixing with cerabone®
Mixing of maxgraft® granules with cerabone® combines the advantages of both materials. The biological potential of maxgraft® and the long-term volume stability of cerabone® lead to fast regeneration of strong vital bone.
Guided Bone Regeneration – Use of a barrier membrane
maxgraft® granules should be covered with a barrier membrane (e.g. Jason® membrane, collprotect® membrane) for the purpose of controlled bone regeneration; this prevents premature resorption and ingrowth of soft tissue.
Wound closure
Consideration of the soft tissue situation prior to surgery is necessary. The wound must be closed tension-free and saliva-proof. Overlapping mobilization of the soft tissue before suturing should be possible.
Reentry
Depending on the defect morphology, the healing time of maxgraft® granules is 3-4 months (e.g. socket preservation, smaller bone defects, periodontal defects). Following healing, reentry with implant placement should be performed promptly to prevent degradation.
- Natural mineralized collagen
- Three-dimensional pore network and rough surface
- Osteoconductive scaffold which supports natural and controlled remodeling3,4
Product Specifications

maxgraft® cancellous granules
| Art.-No. | Particle Size | Content |
|---|---|---|
| 30005 | < 2.0 mm | 1 × 0.5 ml |
| 30010 | < 2.0 mm | 1 × 1.0 ml |
| 30020 | < 2.0 mm | 1 × 2.0 ml |
| 30040 | < 2.0 mm | 1 × 4.0 ml |
| 30005S | 0.25–1mm | 1 × 0.5 ml |
| 30010S | 0.25–1mm | 1 × 1.0 ml |
| 30020S | 0.25–1mm | 1 × 2.0 ml |
| 30040S | 0.25–1mm | 1 × 4.0 ml |
| 30005L | 1.0–2.0 mm | 1 × 0.5 ml |
| 30010L | 1.0–2.0 mm | 1 × 1.0 ml |
| 30020L | 1.0–2.0 mm | 1 × 2.0 ml |
| 30040L | 1.0–2.0 mm | 1 × 4.0 ml |
maxgraft® cortico-cancellous granules
| Art.-No. | Particle Size | Content |
|---|---|---|
| 31005 | < 2.0 mm | 1 × 0.5 ml |
| 31010 | < 2.0 mm | 1 × 1.0 ml |
| 31020 | < 2.0 mm | 1 × 2.0 ml |
| 31040 | < 2.0 mm | 1 × 4.0 ml |
| 31005S | 0.25–1mm | 1 × 0.5 ml |
| 31010S | 0.25–1mm | 1 × 1.0 ml |
| 31020S | 0.25–1mm | 1 × 2.0 ml |
| 31040S | 0.25–1mm | 1 × 4.0 ml |
| 31005L | 1.0–2.0 mm | 1 × 0.5 ml |
| 31010L | 1.0–2.0 mm | 1 × 1.0 ml |
| 31020L | 1.0–2.0 mm | 1 × 2.0 ml |
| 31040L | 1.0–2.0 mm | 1 × 4.0 ml |
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
RELATED PRODUCTS

maxgraft® cortico
BONE AUGMENTATION WITH THE SHELL TECHNIQUE
A prefabricated cortical bone strut from human donor bone.

maxgraft® blocks
PROCESSED HUMAN ALLOGRAFT
Allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process
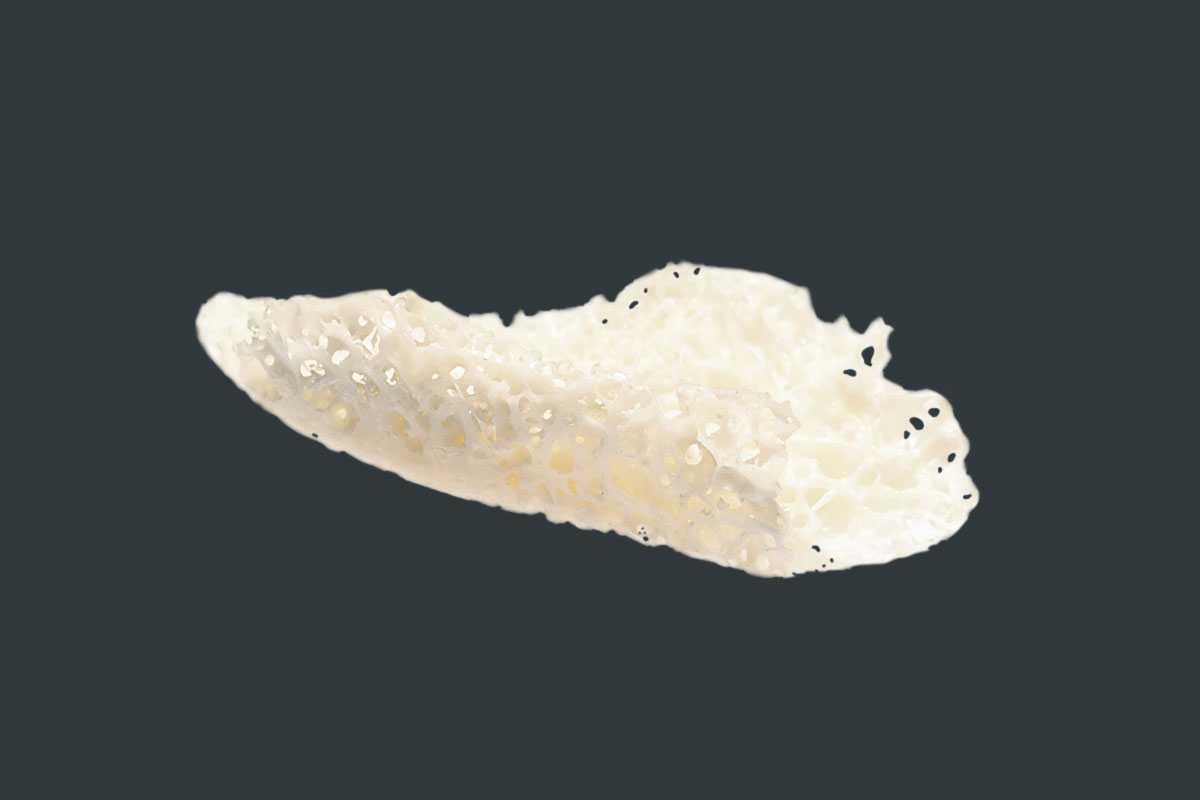
maxgraft® bonebuilder
CUSTOMIZED ALLOGENIC BONE BLOCK
A cancellous allogenic bone block made from human donor bone, individually adapted to the patient defect, which is prepared by Cells+Tissuebank Austria in a special purification process.