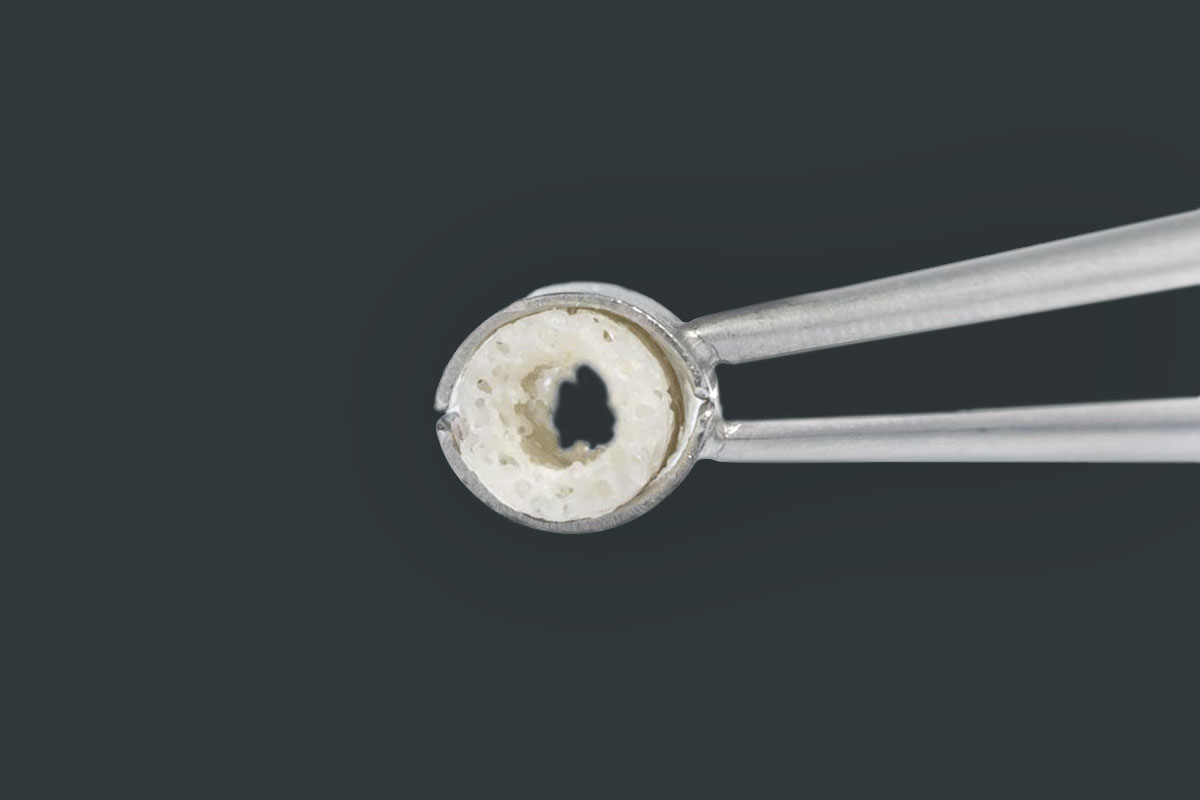
maxgraft® bonering
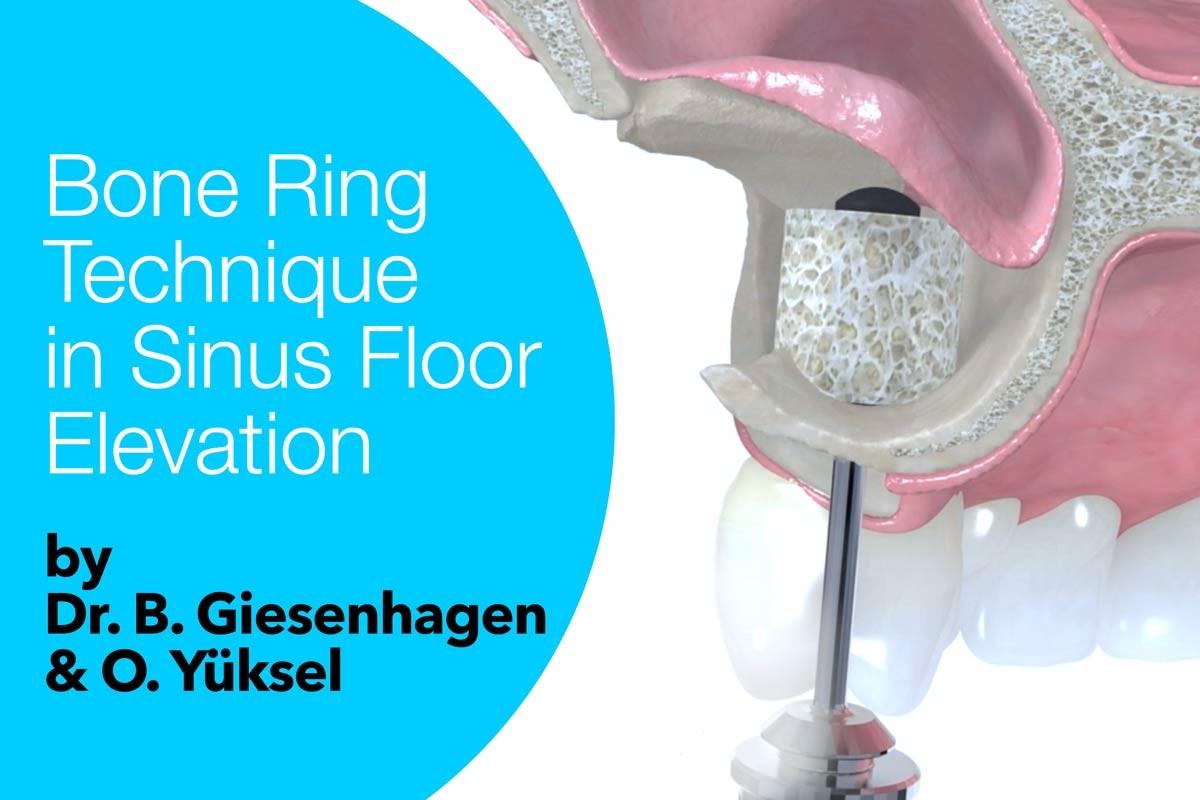
Simultaneous bone augmentation and implant placement

patient comfort
SHORTER TREATMENT TIMES
One-stage procedure
maxgraft® bonering is a prefabricated cancellous ring made from processed human donor bone. maxgraft® bonering enables the implant placement and bone augmentation in one step1 .
FAST RECONSTRUCTION OF BONE
With the Bone Ring Technique, the treatment time can be shortened by several months, as a second operation for implant placement is avoided. The bony integration of the bone ring and the implant takes place over the surrounding vital bone. After a six-month healing period, reopening and prosthetic restoration can take place. The use of an allogenic bone ring instead of the patient’s own bone can increase patient comfort. The risks associated with autogenous bone harvesting, such as donor site morbidity, increased risk of infection and postoperative pain, can be avoided or prevented1,2.
HIGH PATIENT ACCEPTANCE – SHORTER TREATMENT TIMES
Due to its high biological regenerative capacity and complete remodeling, maxgraft® represents an alternative to the patient’s own bone. The need for a second surgical site is eliminated, surgical time is shortened, and postoperative pain and morbidity for the patient are significantly reduced.
IMPLANTOLOGY, ORAL AND CMF SURGERY
- Vertical augmentation (three-dimensional defects with low-grade horizontal augmentation)
- Single tooth gap
- Edentulous space
- Sinus lift (4mm to 1mm residual bone height)
Contraindications:
- Very narrow parallel-walled alveolar ridge
- Less than 1mm residual bone height in the sinus
Rehydration
The production process of maxgraft® preserves the natural collagen and a residual moisture of <10%. Nevertheless it is recommended to rehydrate maxgraft® bonering before use (10 min in saline solution). Rehydration in saline solution leads to improved flexibility of the ring (especially for the 7 mm ring), therefore it is less prone to fracture and can be more easily adapted to the defect area.
Preparation of the bone bed with the maxgraft® bonering surgical kit
With the maxgraft® bonering surgical kit, all instruments required for the application of the bone ring technique are provided. For more detailed information on the surgical procedure, please refer to the maxgraft® bonering surgical guide.
Combination with cerabone®
The use of maxgraft® bonering and cerabone® combines the advantages of allogeneic and bovine bone graft substitutes – the biological potential of maxgraft® and the volume stability of cerabone®, which protects against resorption and improves the esthetic result.
Guided Bone Regeneration – Use of a barrier membrane
maxgraft® bonering should be covered with a membrane with a sufficient long barrier function (e.g. Jason® membrane) to achieve controlled bone regeneration and prevent soft tissue ingrowth.
Wound closure
Consideration of the soft tissue situation prior to surgery is necessary. The wound must be closed tension-free. Overlapping mobilization of the soft tissue before suturing should be possible.
Reentry
maxgraft® bonering is fixed on the bone with primary stability using a suitable implant. The prosthetic restoration of the implant should take place approximately 6 months after implantation to avoid resorption due to insufficient mechanical loading. The appropriate time for implant uncovering is determined individually by the practitioner.
- One-stage procedure: simultaneous bone augmentation and implant placement
- Cancellous bone ring, available in different sizes (depending on implant)
- Innovative treatment concept
- Osteoconductive properties that support natural and controlled remodeling

Product Specifications
maxgraft® bonering 3.3
Recommended for implant diameters from 3.3 – 3.5 mm
| Art.-No. | Dimension | Content |
|---|---|---|
| 33160 | cancellous ring, ø 6 mm, height 10 mm
|
1 x |
| 33170 | cancellous ring, ø 7 mm, height 10 mm
|
1 x |
maxgraft® bonering 4.1
Recommended for implant diameters from 4.1 – 4.5 mm
| Art.-No. | Dimension | Content |
|---|---|---|
| 33174 | cancellous ring, ø 7 mm, height 10 mm
|
1 x |
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
RELATED PRODUCTS

cerabone®
100% pure bone mineral
A 100% pure bone mineral of bovine origin manufactured by a unique 1200°C production process.
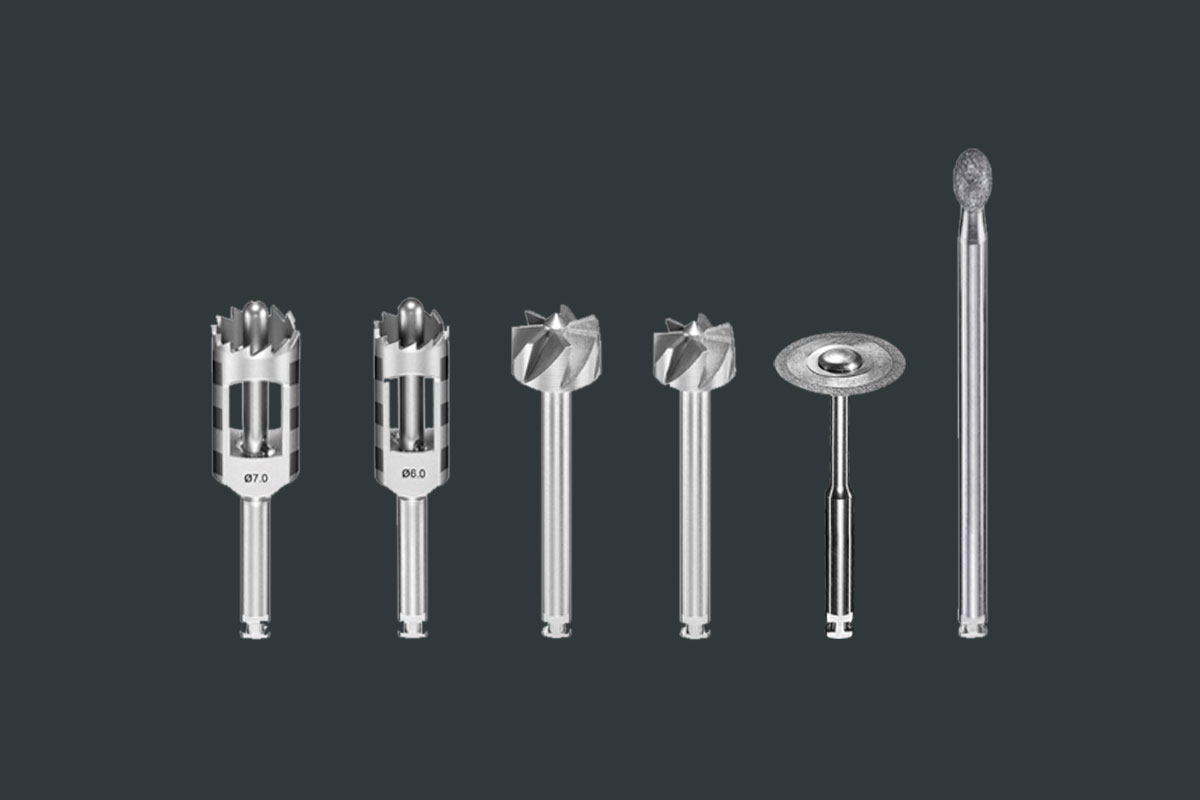
maxgraft® bonering surgical kit
All necessary instruments for maxgraft® bonering
With this surgical kit, botiss biomaterials provides all necessary instruments to apply the maxgraft® bonering.

Jason® membrane
Native pericardium membrane for GBR/GTR
A native collagen membrane obtained from porcine pericardium, offering multi-directional strength and tear resistance.