NOVAMag® SHIELD
RESORBABLE MAGNESIUM SHIELD

COMPLETELY RESORBABLE
BUT VOLUME STABLE
REDUCED INVASIVENESS
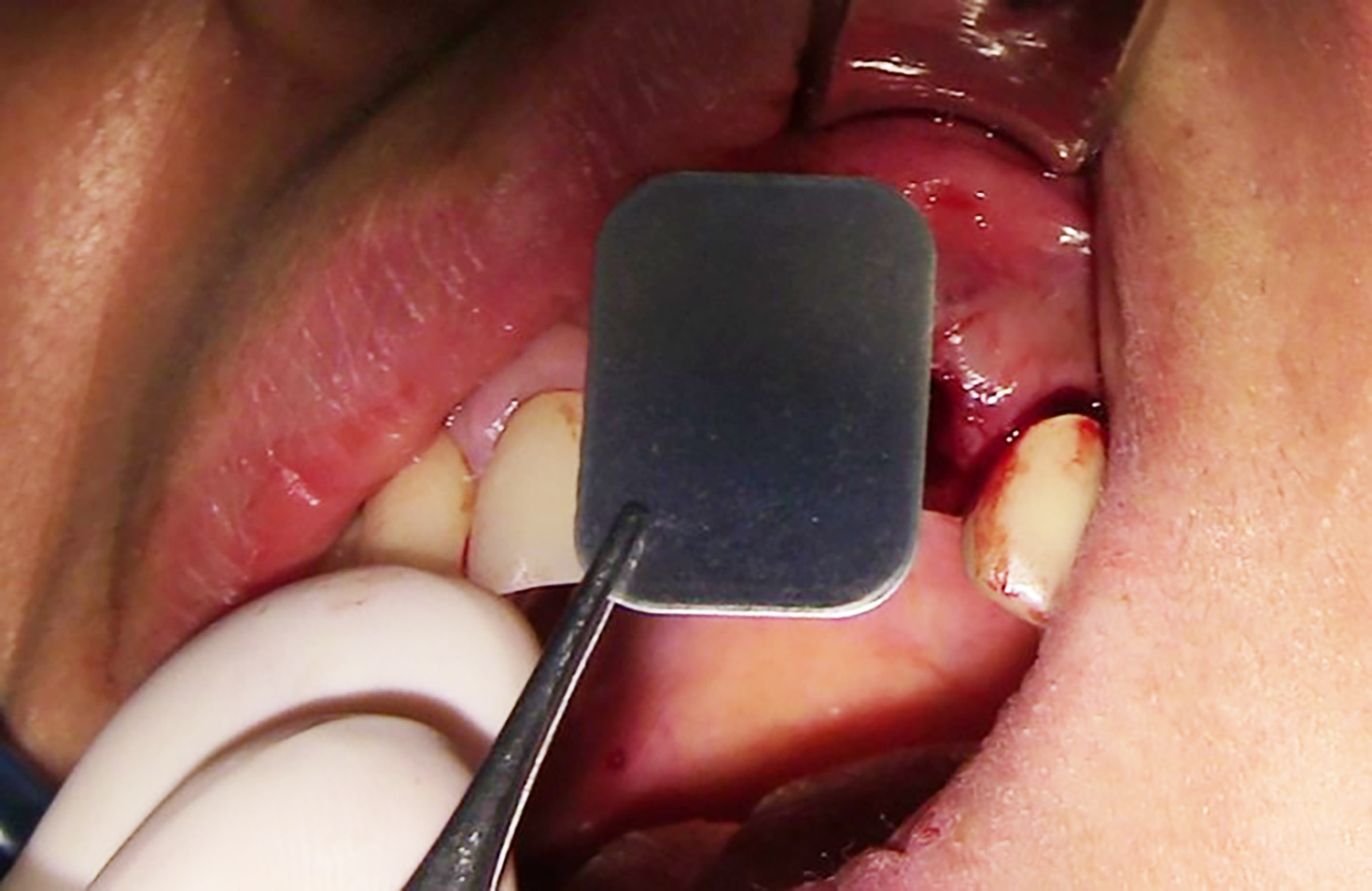
The fully resorbable NOVAMag® SHIELD introduces a pioneering solution for managing buccal and palatal wall defects. Utilizing a flapless approach without the need for membrane fixation, NOVAMag® SHIELD can be positioned between the soft tissue and bone, thus providing support to the bone graft material while reinforcing the structural integrity of the augmented site. The approach reduces the need for extensive bone grafting and promotes the preservation of the natural bone contour. By reconstructing alveolar walls, NOVAMag® SHIELD minimizes the risk of resorption in the buccal and palatal plates, ultimately enhancing the success of implant procedures.
Mechanically Strong and resorbable
The NOVAMag® SHIELD is produced from pure magnesium metal. Magnesium is a biodegradable metal that is resorbed by the human body without toxic residuals. During the degradation process magnesium ions (Mg2+) are released. They are a naturally occurring component in the human body and are responsible for many physiological processes. Due to the inherent properties of magnesium metal, the NOVAMag® SHIELD provides a mechanically strong yet degradable material option for bone augmentation surgeries.
You are currently viewing a placeholder content from Vimeo. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information- in case of surgical bone defects and bone wall defects
- in the context of ridge preservation
- in extraction sockets after tooth extractions
- in case of GBR in conjunction with immediate or delayed implant placement
Thickness: 140 ± 20 μm
- Biodegradable metal
- Produced from pure magnesium
- Synthetic and fully resorbable (within 2-4 months)
- Volume stable
- No removal surgery necessary resulting in fewer surgical interventions and less chair time
- Bone Preservation: Maintaining the existing bone structure, reducing the need for extensive bone grafting and complex augmentation procedures.
- Ridge Reconstruction: Preservation of the original bone contour and support of both hard and soft tissues regeneration, resulting in a robust and voluminous ridge that can securely accommodate dental implants.
- Simplified Procedure & Easy Application: By eliminating the need for flap preparation with additional incisions, fixation, and membrane removal, the surgical process is simplified, making it less invasive for the patient and more efficient for the clinician.
PRODUCTORDER
Do you have your practice in Germany or Austria? Then order directly here:
If your practice is located outside of Germany and Austria, please contact our product management.
To stay up to date, please register here for our newsletter.
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
FAQ
RELATED PRODUCTS

cerabone® plus
Natural bovine bone substitute material with hyaluronate
Combines the established bovine bone grafting material cerabone® with the well-known properties of hyaluronic acid.

mucoderm®
Acellular dermal collagen matrix
Acellular collagen matrix that offers a safe alternative to autologous soft tissue transplants in a diverse range of soft tissue grafting indications.
maxgraft® granules
Processed human allograft
Allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process