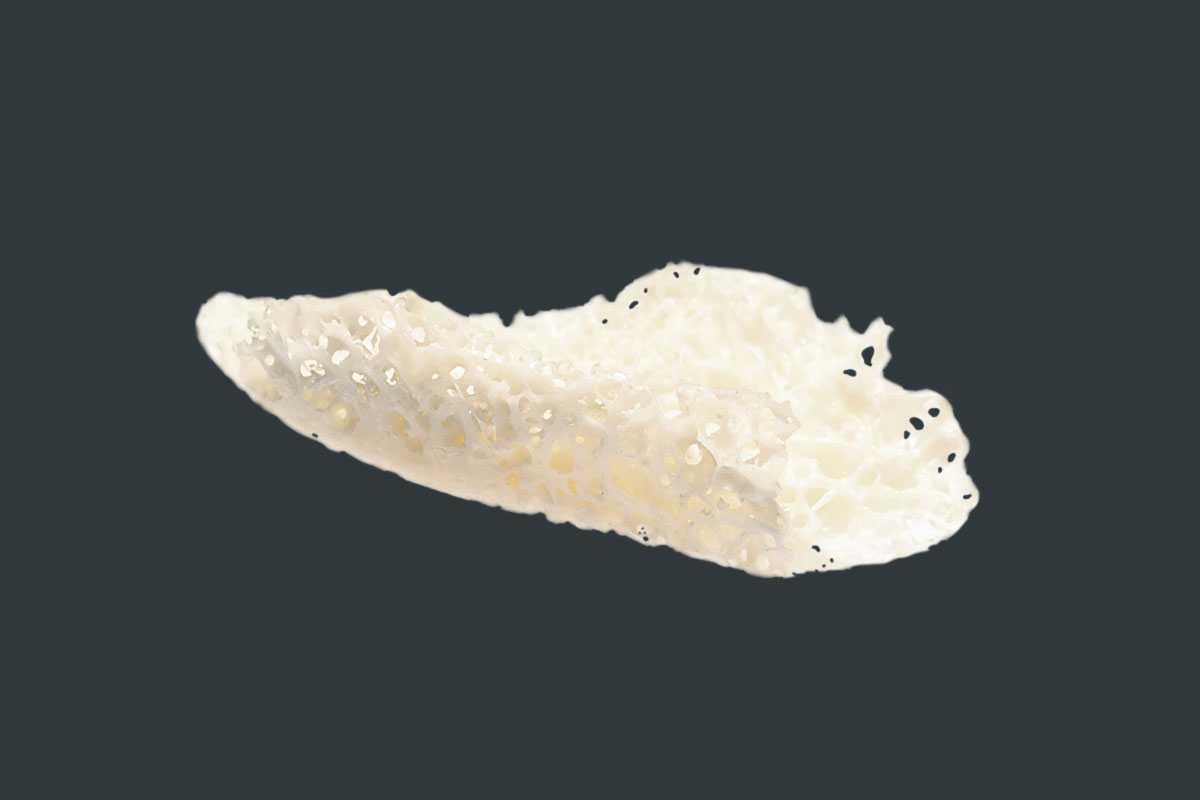
maxgraft® bonebuilder
CUSTOMIZED ALLOGENIC BONE BLOCK

high precision
CAD/CAM TECHNOLOGY
SHORTER TREATMENT TIMES
maxgraft® bonebuilder is a cancellous allogenic bone block made from human donor bone, individually adapted to the patient defect, which is prepared by Cells+Tissuebank Austria in a special purification process (Allotec® process). The three-dimensional fit is planned using state-of-the-art 3D CAD/CAM technology and the block can be inserted with high precision. Surgical time can be significantly shortened and the risk of infection significantly reduced. Increased patient comfort without additional pain and without donor site morbidity is the result1,2.
BLOCK DESIGN WITH CAD/CAM TECHNOLOGY
By planning the ideal fit preoperatively with 3D CAD/CAM technology, optimal contact of the block to the recipient bed can be achieved. Manual adjustment of the cancellous block is usually no longer required. The maxgraft® bonebuilder planning takes place in an interactive exchange between the clinical user and the botiss CAD designers. For more information on customization, please visit the maxgraft® bonebuilder website.
HIGH PATIENT ACCEPTANCE – SHORTER TREATMENT TIMES
Due to its high biological regenerative capacity and complete remodeling, maxgraft® represents an alternative to the patient’s own bone. The need for a second surgical site is eliminated, surgical time is shortened, and postoperative pain and morbidity for the patient are significantly reduced.
IMPLANTOLOGY, ORAL AND CMF SURGERY
- Horizontal and vertical augmentation
- Complex bone defects
Data quality and block fit
For 3D planning, the CT/CBCT data should be as up-to-date as possible to achieve the best possible result. Depending on the imaging and possible bone changes during the manufacturing process, slight deviations in accuracy of fit cannot be completely ruled out. Preparation of suitable instruments for making manual adjustments is recommended.
Rehydration
The production process of maxgraft® preserves the natural collagen and a residual moisture of <10%. Nevertheless, rehydration of maxgraft® bonebuilder is recommended (10 minutes in physiological saline solution). Rehydration can improve the adaptability of the material (especially with large blocks) to the defect3. maxgraft® bonebuilder can also be briefly rehydrated in a disposable syringe under vacuum in physiological saline solution (technique according to Dr. Blume).
Fixation – Lag screw technique
maxgraft® bonebuilder must be stabilized to avoid any micro-movement and thus ensure good healing. Direct contact between the local bone and maxgraft® bonebuilder ensures safe incorporation and rapid regeneration. With the lag screw technique, a gliding hole is drilled into the bone block (drill diameter corresponds to screw diameter), while a drill with a minimally smaller diameter is used for the local bone. Before fixation, a suitable head space for the screws should be created, e.g. with a diamond burr, to avoid soft tissue irritation due to protruding screw heads. Flat-headed osteosynthesis screws are recommended to avoid sharp edges.
Combination with cerabone® or maxgraft® granules
Bone substitute material (e.g. cerabone® or maxgraft® granules) can be used at the margins of the maxgraft® bonebuilder for contouring. Volume-stable bone substitute materials such as cerabone® can also be used as resorption protection, e.g. particularly in the aesthetic zone.
Guided Bone Regeneration – Use of a barrier membrane
maxgraft® bonebuilder should be covered with a membrane with sufficient long barrier function (e.g. Jason® membrane) to achieve guided bone regeneration and prevent soft tissue ingrowth.
Wound closure
Consideration of the soft tissue situation prior surgery is necessary. The wound must be closed tension-free. Overlapping mobilization of the soft tissue should be possible before suturing.
Reentry
The healing time for maxgraft® bonebuilder is approx. six months (depending on the location, geometry and extent of the defect). During reentry, the osteosynthesis screws are removed.
- Natural mineralized collagen
- Preserved trabecular, cancellous structure of human bone
- Osteoconductive properties that support natural and controlled remodeling
- Max. size 23 x 13 x 13 mm (for larger defects, several blocks can be designed per patient)

Product Specifications
maxgraft® bonebuilder
| Art.-No. | Content |
|---|---|
| PMIa | Individual planning and production of a bone block max. dimensions 23 x 13 x 13 mm |
| PMIa 2 | maxgraft® bonebuilder, additional block(s) for this patient |
bonebuilder dummy
| Art.-No. | Content |
|---|---|
| 32100 | Individual 3D-printed model of the patient’s defect and the planned maxgraft® bonebuilder for demonstration purposes made of plastic |
Distribution
With our international network of distribution partners, we are near you in over 100 countries worldwide. In addition to our 360° productportfolio, we offer service, scientific advice and exchange, training and events directly on site from a single source.
Find a distribution partner near you:
RELATED PRODUCTS

cerabone®
100% pure bone mineral
A 100% pure bone mineral of bovine origin manufactured by a unique 1200°C production process.

Jason® membrane
Native pericardium membrane for GBR/GTR
A native collagen membrane obtained from porcine pericardium, offering multi-directional strength and tear resistance.

maxgraft® blocks
Processed human allograft
Allograft bone substitute from human donor bone, processed by the Cells+Tissuebank Austria with a special cleaning process