ARTICLE
To commemorate the receipt of the CE marking certification of cerabone® 15 years ago, five leading experts, with extensive experience in the field of dental bone regeneration and a solid clinical experience with cerabone®, gathered to discuss all relevant aspects of the clinical performance of the bone grafting material. The debate predominantly focused on the clinical indications of cerabone® and its handling.
The goal of the meeting was to develop an indication-based guideline for the clinical use of cerabone®, i.e. which basic principles should be followed when using cerabone® for different bone augmentation procedures.
Experts invited were Assoc. Prof. Dr. Ziv Mazor (Raanana, Israel), Dr. Alessandro Rossi (University of Milan, Italy), Dr. Pedro Lázaro Calvo (Madrid, Spain), PD Dr. mult. Peer Kämmerer (University Medical Centre Rostock, Germany) and Dr. Marko Blašković (Rijeka, Croatia).cerabone® is a natural cancellous bovine bone grafting biomaterial. The raw material undergoes a step-wise heating process (up to >1200 °C), which removes all organic components, including potential bacteria, viruses and prions, and therefore minimizing the risk of immunological reactions or disease transmission (Tadic & Epple 2004, Murugan et al. 2003). Heating above 800 °C ensures a complete inactivation of the infectivity of potential prions (Brown et al. 2000). The CE certification of cerabone® was issued in 2002 and the product has been on the dental market since 2006. As of 2016, more than 650.000 patients worldwide have been successfully treated with cerabone® in different indications in the fields of implantology, oral and cranio-maxillofacial surgery and periodontology. cerabone® is composed of pure bone mineral (Tadic & Epple 2004) with a high porosity of ~65 – 80% and a mean pore size distribution of ~600 – 900 μm, thus strongly resembling the human mineralized bone matrix (Seidel & Dingeldein 2004, Tadic et al. 2004). As such, cerabone® provides an appropriate scaffold for the adherence and migration of osteogenic and blood vessel-forming cells, which in turn promotes bone regeneration (Rothamel et al. 2009). Clinically, cerabone® has demonstrated complete osseous cellular integration and exceptional long-term volume stability (Tawil et al. 2016, Fienitz et al. 2016, Panagiotou et al. 2015, Lorean et al. 2014, Riachi et al. 2012, Artzi et al. 2012, Rothamel et al. 2011).
Figure 1: Left: Architecture of a single cerabone® particle illustrating its trabecular basic structure. Central: Presentation of the surface structure of cerabone® demonstrating the preservation of the natural micro structure. Right: Cross-section of a cerabone® particle showing the preservation of the lamellar basic structure.
Clinical use of cerabone®
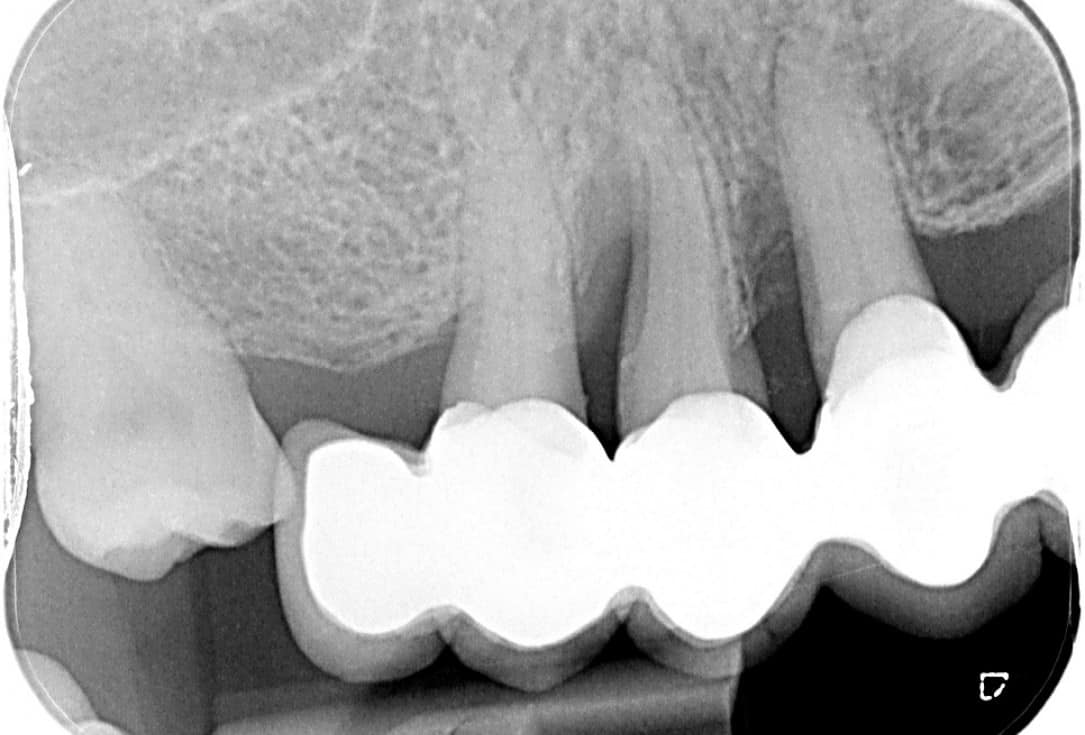
After a stimulating discussion, the experts came to the conclusion that cerabone® is the biomaterial of choice for 3 major clinical indications, i.e. sinus/nasal floor elevation, horizontal (and possibly also vertical) Guided Bone Regeneration (GBR) and socket/ridge preservation, the latter specifically in combination with allogenic bone grafts. In addition, cerabone® may be used for covering of autologous/allogenic bone blocks/rings to prevent uncontrolled resorption. Clinical data on cerabone® with 4 – 15 years follow-up point to the remarkable long-term stability in the above-mentioned indications.For sinus/nasal floor elevation, cerabone® can be used as the sole grafting material (Tawil et al. 2016, Lorean et al. 2014, Mazor et al. 2010). The experts highlighted the good radio-opacity of cerabone®, which is beneficial especially in this indication, since the healing course and the graft stability can be easily monitored. In addition, it was suggested that both granule sizes can be used to augment the maxillary sinus; however, the large particles, i.e. 1 – 2 mm, may provide more inter-particular space enabling the formation of more new bone between the particles and a more stable situation of bigger defects. The re-entry in this indication should be performed earliest 6 months post-surgery depending on the size and morphology of the sinus. Wider and anatomically complex sinus cavities may benefit from a longer healing time of up to 1 year. For lateral approaches, the expert group recommended to cover the bone window with a resorbable membrane; other methods, such as the bone window repositioning technique and covering with PRF (Platelet Rich Fibrin) membranes, may also be suitable to protect the grafted sinus cavity. Notably, Dr. Kämmerer stated that he observed no differences in the clinical outcome when the grafted sinus was covered by a membrane or just by the flap. For the internal sinus approach, a putty may be preferred as it provides a more comfortable handling and helps to minimize the risk of a perforation of the Schneiderian membrane.
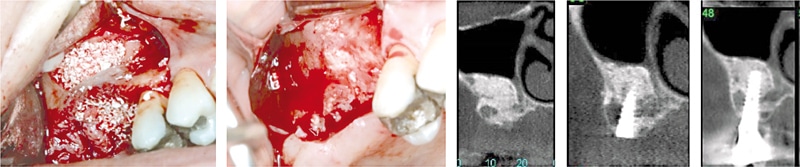
Figure 2: Two-stage sinus floor elevation with cerabone®. Re-entry and implant placement 6 months post-operative. X-ray control at 12 months and 6 years post-op (rightmost) reveals a stable clinical situation. (By courtesy of Dr. Ziv Mazor)
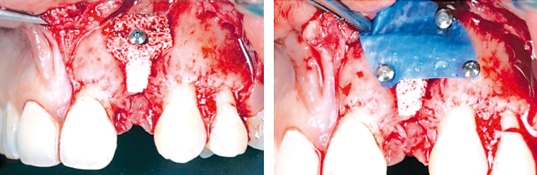
Owing to its ability to create convexity and to provide a long-lasting, stable scaffold with nearly no resorption, the experts concluded that cerabone® can be considered the preferred bone grafting material for GBR procedures (Kojasteh et al. 2016). The expert group stressed the small cerabone granules (0.5 – 1 mm) to be preferable in this specific indication, since they are specifically suitable for contouring in the aesthetic area. Furthermore, the expert group agreed that horizontal GBR with cerabone® covered with Jason® membrane is the ideal treatment concept (Forna et al. 2017). Dr. Mazor suggested to ensure a stable fixation of the granules with the membrane and the immobilization of the particles with PRF or hyaluronic acid – this can help avoiding particle migration. Dr. Blašković stressed the importance of using tenting screws for lateral GBR to create and maintain space. He emphasized that the use of tenting screws or pins are specifically indicated to regenerate bone defects outside the ridge contour. In such situations the membrane should be fixed with pins or sutures to ensure immobilization of the cerabone® granules. In challenging situations, i.e. when large defects have to be regenerated, the combination of the two techniques, i.e. tenting screws and pins for membrane fixation, could be advantageous. For simultaneous implant placement, the sandwich technique should employed, i.e. autologous bone chips are placed on the implant surface covered by a layer of small cerabone® granules (Steigmann et al. 2009, Wang et al. 2004). The experts pointed out that vertical GBR with cerabone® may be limited; only 1 – 2 mm bone gain may be achieved vertically while extensive vertical augmentation should only be performed with a more stable, space maintaining membrane and a mixture of xeno- and auto-/allograft. Accordingly, mixing cerabone® with autologous or even allogenic bone (maxgraft®) may provide the best results for major defects.
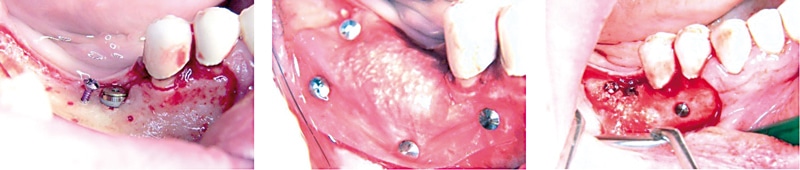
Figure 3: Vertical/horizontal ridge augmentation and treatment of a periimplant defect with cerabone® mixed with autologous bone by means of a tenting screw. Augmented area was covered by a resorbable membrane (Jason® membrane) immobilized by pins. Re-entry 6 months post-operative (rightmost). (By courtesy of Dr. Marko Blašković)
The expert group stated that cerabone® is the bone grafting biomaterial of choice for socket or ridge preservation, if no implant placement is planned within 1 – 2 years following tooth extraction (pontic side development). The material remains within the augmentation area embedded into newly formed bone and therefore helps to maintain the volume and shape of the alveolar ridge. Moreover, if late implantation is planned, e.g. tooth extraction with following orthodontic treatment, cerabone® may be the ideal bone graft to preserve the dimensions of the ridge. To immobilize the granules, cerabone® should always be covered by a barrier membrane or a collagen fleece depending on the integrity and morphology of the alveolar socket. According to Dr. Kämmerer, if cerabone® is used as the sole grafting material, re-entry should be performed earliest 6 months post-operative according to the concept of late implantation. If earlier implant placement is desired, mixing cerabone® with auto- or allograft (maxgraft®) in the range of 50:50 – 80:20 should be considered. The preserved collagen content of allografts can promote an accelerated bone remodeling.
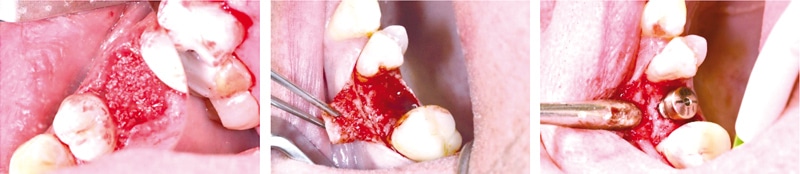
Figure 4: Socket preservation with cerabone®. The augmented socket was covered by a resorbable membrane and the flap was closed. Re-entry and implant placement 6 months post-operative. (By courtesy of PD Dr. mult. Peer Kämmerer)
The high temperature treatment of the bovine bone makes cerabone® resistant to osteoclastic resorption. Thus, the material degrades very slowly under physiological conditions, offering remarkable volume stability. According to Dr. Lázaro Calvo and Dr. Blašković, cerabone® is particularly suitable to prevent uncontrolled resorption of autogenous or allogenic bone grafts (Monje et al. 2014, Nissan et al. 2011). The auto- or allograft should be covered by a relatively thin layer of cerabone® granules before membrane placement and flap closure. Preferably, small particles should be used in this indication, since they ensure a better contouring of the distal and mesial aspects of the bone block/ring.
In summary, cerabone® is a reliable and versatile bone substitute material, especially beneficial in indications where a permanent structural support is required or valuable. cerabone® can be used as sole grafting material in sinus floor elevation (lateral approach) and socket preservation. In contrast, a mixture of cerabone® with autologous bone or allogenic bone grafts (maxgraft®) is recommended for ridge augmentations. These different biomaterials (cerabone® and maxgraft®) complement each other, maxgraft® promoting a biological bone remodeling, cerabone® providing permanent structural support and stability.
Handling and application of cerabone® granules
The high temperature treatment of the bovine bone removes any organic components or remnants of water, converting cerabone® into a non-resorbable, pure bone apatite (Tadic & Epple 2004). cerabone® granules may appear hard and sometimes sharp, depending on the individual experience with different biomaterials. Nevertheless, the expert group agreed that this perception has no clinical relevance. Moreover, the hardness of the particles can even be advantageous, since it can provide a very stable bone bed for later implantation. The experts, however, stressed that clinical users should, as for every biomaterial, follow a few important rules when working with cerabone® in the daily practice. In general, small granules are preferred in all relevant clinical indications, in particular in GBR procedures as they offer a better handling and contouring. In addition, the relative perception of the hardness of the particles is lower as compared to the larger ones. According to Dr. Rossi, the small granules may also give better surface contouring, especially when augmenting in the aesthetic region. This property is also favorable when the granules are used to cover autologous/allogenic bone blocks and to fill remaining gaps when a block grafting is performed. Large cerabone® granules are mainly indicated for lateral sinus floor elevation, as they offer larger intra-particular space. Although not clearly proven yet, clinical studies in sinus floor elevation point to a more efficient bone formation when larger granules are used (Testori et al. 2013).
Interestingly, the experts agreed that much smaller particles, i.e. < 0.5 mm are not required in the daily practice and might be even disadvantageous as the space between very small particles for bone matrix deposition is limited. They may be only suitable for the treatment of periodontal bone defects. It should be remarked that smaller granules can also be prepared by the clinician by gently grinding the particles.
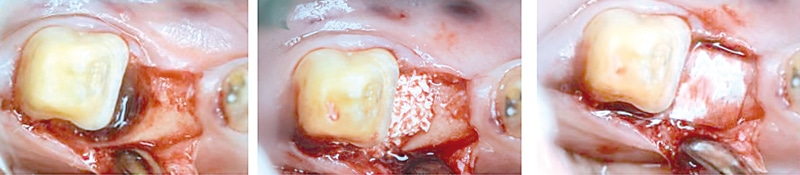
Figure 5: Treatment of a periodontal intrabony defect with small cerabone® granules covered by a resorbable membrane (Jason® membrane). (By courtesy of Dr. Dávid Botond Hangyási)
Prior to application, cerabone® granules should be rehydrated in blood or sterile saline solution. This will facilitate the handling and application of the granules, as they will adhere to each other. cerabone® is a particularly hydrophilic bone grafting material, which takes up fluids very quickly, because of its ultra-porous nature. In particular, the micro-pores of the bone mineral scaffold of cerabone® promote a rapid blood uptake through capillary effect. A rehydration in blood may be advantageous as serum proteins penetrate the graft, which in turn may improve revascularization after implantation.
Regarding the application of cerabone®, the expert group stressed that a gentle placement of the material is crucial to avoid excessive compression of the particles and to provide space for the regenerative process. Excessive compression will affect the natural interconnected porosity of cerabone® and may result in a limited revascularization and osseointegration.
Another critical factor determining the success of the clinical outcome is the immobilization of the cerabone® particles. Fixation and covering of the granules by a membrane is of utmost importance; substances like PRF or hyaluronic acid may be used additionally to contain the graft. When treating defects outside the ridge contour, a fixation of the membrane by pins or screws can be advantageous. The expert group agreed that the Jason® membrane is the barrier membrane of choice for horizontal GBR; for vertical GBR, (reinforced) non-resorbable membranes, titanium meshes or tenting screws, are recommended to stabilize the graft. Further, Dr. Lázaro Calvo stressed that the use of cerabone® blocks may lead to a longer healing time and therefore should only be covered by a non-resorbable membrane.
Figure 6: cerabone® block used for horizontal ridge augmentation, fixed with a miniscrew and covered by a non-resorbable membrane (permamem®). (By courtesy of Dr. Pedro Lázaro Calvo)
The experts emphasized that depending on the indication, cerabone® should be mixed with autologous/allogenic bone. This will give additional biological information and reduce the time needed for graft integration. In socket/ridge preservation mixing it with up to 80% of maxgraft® is recommended, depending on the patience and financial capabilities of the patient. The use of cerabone® alone can be recommended for sockets when later implantation is not planned (see above). Dr. Mazor stressed the sandwich technique (layering of autologous bone and cerabone®) to be the preferred concept for buccal augmentation; for horizontal GBR, it may be preferable to use a mixture with auto-/allograft in the ratio of 50:50. Accordingly, augmented defects require a shorter or longer healing time. In general, re-entry after horizontal GBR and socket/ridge preservation should be performed about 6 months post-surgery, if cerabone® is used as the sole grafting material. If using allografts and cerabone® in the ratio of 50:50 a re-entry after already 4 months is feasible. Augmentation of maxillary sinuses usually do not require addition of auto-/allograft and accordingly should be re-entered earliest 6 months post-surgery.Several studies have tested cerabone® in comparison with that of the bovine-derived bone grafting material BioOss®, demonstrating no significant difference in the clinical outcome (Fienitz et al. 2016, Panagiotou et al. 2015, Riachi et al. 2012). Both materials consist of pure bone mineral without any organic phase. However, they differ in their production processes and hence in some biochemical characteristics. To ensure the safety of the material, cerabone® is processed by a high temperature treatment (>1200°C). This unique manufacturing process leads to a higher crystallinity and phase purity compared to BioOss®, which shows a 3% residual amount of water (Tadic & Epple 2004). In addition, its mineral phase contains carbonate which is absent in cerabone® (Tadic & Epple 2004). Due to a higher solubility, incorporated carbonate enhances the biodegradation of a grafting material. Therefore, cerabone® may provide a higher long-term volume stability compared to BioOss® (Riachi et al. 2012).
The high crystallinity is the reason that cerabone® particles appear harder than BioOss® particles. The expert group pointed out that also the smaller particle size of BioOss® (0.25 – 1 mm) as compared to that of cerabone® (0.5 – 1 mm) has an impact on the handling and material perception.
The experts stated that due to the hard particles bone augmented with cerabone® is much harder compared to bone augmented with other materials and that cerabone® offers a higher stability under pressure. Though, the harder character of the cerabone® granules may facilitate their migration through the mucosa, thus particles may be visible in the flap at re-entry or in the mucosa during the healing course. However, the expert group stated that separated granules found in the soft tissue are considered unproblematic for the patient and the healing process, as their appearance is not associated with an inflammatory reaction neither does it affect the regenerative process. Usually, particles visible in the superficial part of the mucosa will grow out. Particles found on the inner aspect of the flap, visible at time of re-entry, can be removed with forceps. In general, particle migration can be avoided or minimized by good immobilization with a barrier membrane or by good compaction using PRF (sticky bone) or suchlike.
To summarize, the handling and application of the cerabone® granules is convenient in particular attributed to their hydrophilic character. The individual impression of the structure of the granules can be quite different, specifically if someone has worked with other bone grafts before. For a successful outcome, immobilization of the granules at the augmentation site is considered most essential. Occasionally migrating particles found in the flap can simply be removed; however, the augmented site will show a well osseous integration of the graft suitable for implant placement.
Summary
Based on their long-term experiences with cerabone®, the experts conclude that cerabone® is a reliable bone grafting material with an outstanding long-term stability. As such, it is suitable to maintain the dimensions of the alveolar ridge for implant-supported prosthesis over decades. They also emphasized that special care has to be taken for the application of the granules, i.e. good rehydration, gentle compression, and, most importantly, sufficient containment and immobilization of the graft.
Regarding the handling properties, cerabone® granules may appear harder because of the high-temperature treatment of the bovine bone during production; the larger average particle size may also contribute to this. Nonetheless, the expert group agreed on the excellent handling characteristics and hydrophilic properties of cerabone®.
Notably, it was emphasized that the available clinical data are solid enough to demonstrate that cerabone® is at least non-inferior to BioOss® with regard to both handling and clinical performance.
Future studies focusing on GBR procedures with different membranes or on ridge preservation with a non-resorbable membrane may be of value.
References
- Artzi Z, Wasersprung N, Weinreb M, Steigmann M, Prasad HS, Tsesis I. Effect of guided tissue regeneration on newly formed bone and cementum in periapical tissue healing after endodontic surgery: an in vivo study in the cat. J Endod. 2012 Feb;38(2):163-9.
- Ayna M, Açil Y, Gulses A. Fate of a Bovine-Derived Xenograft in Maxillary Sinus Floor Elevation After 14 Years: Histologic and Radiologic Analysis. Int J Periodontics Restorative Dent. 2015 Jul-Aug;35(4):541-7.
- Browaeys H, Bouvry P, De Bruyn H. A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Relat Res. 2007 Sep;9(3):166-77.
- Brown P, Rau E H, Johnson B K, Bacote A E, Gibbs C J, Jr., Gajdusek D C New studies on the heat resistance of hamster-adapted scrapie agent: Threshold survival after ashing at 600°C suggests an inorganic template of replication PNAS 2000
- Corbella S, Taschieri S, Weinstein R, Del Fabbro M. Histomorphometric outcomes after lateral sinus floor elevation procedure: a systematic review of the literature and meta-analysis. Clin Oral Implants Res. 2016 Sep;27(9):1106-22.
- Fienitz T, Moses O, Klemm C, Happe A, Ferrari D, Kreppel M, Ormianer Z, Gal M, Rothamel D. Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus augmentation procedures. A prospective, randomized-controlled, clinical multicenter study. Clin Oral Investig. 2016 Apr 30.
- Khojasteh A, Hassani A, Motamedian SR, Saadat S, Alikhasi M. Cortical Bone Augmentation Versus Nerve Lateralization for Treatment of Atrophic Posterior Mandible: A Retrospective Study and Review of Literature. Clin Implant Dent Relat Res. 2016 Apr;18(2):342-59. doi: 10.1111/cid.12317. Epub 2015 Jun 17.
- Khojasteh A, Motamedian SR, Sharifzadeh N, Zadeh HH . The influence of initial alveolar ridge defect morphology on the outcome of implants in augmented atrophic posterior mandible: an exploratory retrospective study. Clin. Oral Impl. Res. 00, 2016, 1–10 doi: 10.1111/clr.12991R.
- Lorean A, Mazor Z, Barbu H, Mijiritsky E, Levin L. Nasal floor elevation combined with dental implant placement: a long-term report of up to 86 months. Int J Oral Maxillofac Implants. 2014 May-Jun;29(3):705-8.
- Mazor Z, Lorean A, Mijiritsky E, Levin L. Nasal floor elevation combined with dental implant placement. Clin Implant Dent Relat Res. 2012 Oct;14(5):768-71. doi: 10.1111/j.1708-8208.2010.00312.x. Epub 2010 Oct 26.
- Monje A, Pikos MA, Chan HL, Suarez F, Gargallo-Albiol J, Hernández-Alfaro F, Galindo-Moreno P, Wang HL. On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. Biomed Res Int. 2014;2014:814578
- Murugan, K. Panduranga Rao, T. S. Sampath Kumar Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties Bulletin of Material Science 2003 Volume 26, Issue 5, pp 523–528
- Nissan J, Ghelfan O, Mardinger O, Calderon S, Chaushu G. Efficacy of cancellous block allograft augmentation prior to implant placement in the posterior atrophic mandible. Clin Implant Dent Relat Res. 2011 Dec;13(4):279-85.
- Riachi F, Naaman N, Tabarani C, Aboelsaad N, Aboushelib MN, Berberi A, Salameh Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int J Dent. 2012;2012:737262.
- Panagiotou D, Özkan Karaca E, Dirikan İpçi Ş, Çakar G, Olgaç V, Yılmaz S. Comparison of two different xenografts in bilateral sinus augmentation: radiographic and histologic findings. Quintessence Int. 2015 Jul-Aug;46(7):611-9.
- Rothamel D, Schwarz F, Smeets R, Happe A, Fienitz T, Mazor Z, Zöller J. Sinus floor elevation using a sintered, natural bone mineral zzi 27(1) 2011
- Rothamel D, Schwarz F, Herten M, Berndsen K, Fienitz T, Ritter L, Dreiseidler T, Zöller J. Impact of Citric Acid Etching on Biocompatibility and Osseous Organisation of a Natural Bovine Bone Mineral: Preliminary Results of an In-Vitro/In-Vivo Study IFMBE Proceedings; Vol 25/11, 2009, 259-262
- Seidel P, Dingeldein E. cerabone® – Bovine based spongiosa ceramic Mat.-wiss. u. Werkstofftech. 2004, 35:208-212
- Steigmann M. Die Grenzen der Implantation mit simultaner gesteuerter Knochenregeneration ZMK, Jg. 25, Ausgabe 11, November 2009
- Tadic D, Beckmann F, Donath T, Epple M. Comparison of different methods for the preparation of porous bone substitution materials and structural investigations by synchrotron l-computer tomography Mat.-wiss. u. Werkstofftech. 2004, 35, No. 4
- Tadic D & Epple M. Sinus floor elevation using a sintered, natural bone mineral. Biomaterials 2004 25:987–994
- Tawil G, Tawil P, Khairallah A. Sinus floor elevation using the lateral approach and bone window repositioning: Clinical and Radiographic Results in 102 treated patients followed from 1 to 5 years. Int J Oral Maxillofac Implants. 2016 Jul-Aug;31(4):827-34.
- Testori T, Wallace SS, Trisi P, Capelli M, Zuffetti F, Del Fabbro M. Effect of xenograft (ABBM) particle size on vital bone formation following maxillary sinus augmentation: a multicenter, randomized, controlled, clinical histomorphometric trial. Int J Periodontics Restorative Dent. 2013 Jul-Aug;33(4):467-75.
- Vanis S, Rheinbach O, Klawonn A, Prymak O, Epple M. Numerical computation of the porosity of bone substitution materials from synchrotron micro computer tomographic data Mat.-wiss. u. Werkstofftech. 2006, 37, No. 6
- Wang HL, Misch C, Neiva RF. “Sandwich” bone augmentation technique: rationale and report of pilot cases. Int J Periodontics Restorative Dent. 2004 Jun;24:232-45.